Abstract THE FULL LUCENT REPORT
Subject
Performance analysis of the corrosion protection of intercept
technology licensed metal substrate storage product
Intercept
Technology is a Lucent Technologies patented and licensed polymer process. The
Technology has been licensed, manufactured, distributed, and sold by our
licensees since 1991. In order to ensure the highest quality production, and
manufacture of Intercept products are maintained, samples of production
materials are regularly retained and tested. These checks, coupled with
periodic inspections and updated improvements in formulations create a
technically advanced product. Periodically, final products are laboratory
tested for static and/or corrosion protection performance.
Coin
storage systems are designed to protect copper, silver and their alloys from
tarnishing. These metals are commonplace in the electronic equipment Lucent
Technologies manufactures. Therefore, it is beneficial for Lucent Technologies
Bell Labs to test their performance of such systems so as to expand our
information base in the matter of the atmospheric corrosion protection of
electronic materials. This report describes such a test for corrosion
protection and its results.
Storage systems for coinage have been
tested for corrosion protection from atmospheric trace sulfur gases. Intercept
Technology significantly outperformed non-Intercept Technology systems.
Background
Copper, silver and
their alloys have been degraded from atmospheric gases from the moment they
were purified and polished more than 5,000 years ago(1). The most abundant corrosive gas is oxygen.
Upon exposure to oxygen copper forms an oxide film of Cu2O, which, is
semitransparent, and self limiting. This oxide grows to approximately 15Å
in one hour to an upper limit of approximately 2 NM at 20° C(2).
Typical copper
degradation occurs when sulfur and water vapor are deposited on the metal
surfaces. Liquid water, sufficient to form an acidic condensate slurry with
sulfur, occurs at relative humidity levels greater than 60%. This slurry
penetrates and breaks protective oxide interstitial grain boundary bonds.
Eventually, sulfur and copper ions form copper sulfide, which, mix into the
oxide, and form directly on the copper surfaces. In very thin layers an overall
darkening will occur at thicknesses as low as 10 nm(3).
Typically, experiments used to mimic
these natural occurring processes utilize water and a corrosive gas. We have
chosen this proven method to evaluate product performance. The gas we wish to
use as a catalyst for the test is hydrogen sulfide. It is abundant in the
atmosphere. It has a natural vapor pressure of 292 psi at STP, is colorless,
and it has an affinity for reacting with copper having a chemical stoichiometry
favoring a Cu reaction as does carbonyl sulfide and three to four times more
than So2(4).
Experimental
H2S levels of 4 ppm
were used in experiments. These have been found to provide an increase in
exposure concentration that follows a linear relationship with total exposure
as shown by Graedel et al(5). Generation of
the atmospheres and exposure chamber was similar to previous work using a
variable length, low pressure permeation tube capped on one end, and connected
to a variable pressure regulated H2S(6)
lecture bottle of technical grade H2S. Continuous monitoring of hydrogen
sulfide (H2S) concentrations were made by a Thermo Electron Model 43 Pulsed
Florescence monitor with precursory catalysis on H2S by platinum reduction.
Temperature measurements were made by a Fluke Model 16 digital
thermometer, and humidity by an EXTECH model 10 humidity meter.
The
test chamber dimensions are 450 x 600 x 600 cm., with a construction of 0.64 cm
thick clear polycarbonate. The chamber has two slotted shelves, and
incorporates a cross feed gas flow system to ensure linear gas concentration
exposures. The air supply line was filtered with an oil separator, an activated
charcoal cartridge, and a 0.5 micron particulate filter.
A continuous
feed water drip maintained the bubbler at 10 cm of water. The water supply was
deionized and triple filtered. Air flow through the chamber was maintained at
10 liters per minute. This flow provided the 162 liter chamber with one volume
exchange per 16.2 minutes.
Following the 90th percentile gas flow rule,
calculations show a complete air exchange occurs at ten times a volume
exchange. Therefore the chamber is completely refreshed every 2 hours 42
minutes.
Coin samples consisted of 1964 to 1980 pennies which all have
a composition of 95% Cu, 5% Zn and, 1964 to 1979 nickels with a composition of
75% Cu and 25% Ni.
The coin samples were degreased with 111
trichloethane, and dried with gaseous nitrogen. They were then placed in
appropriate compartments in the storage media samples.
Evaluation of
the samples was performed with, a LEO 1530 scanning electron microscope for
surface topography, X ray analysis with a Kevex EDXA for elemental analysis,
and a Kodak model 950 digital camera for optical data.
Five types of
storage boxes were evaluated:
- Intercept Album, multipage book with clear plastic covered slot
and an outer cover with Intercept Technology throughout the book.
- Sample
X Album, similar to 1, different vendor, no Intercept
- Sample
Y Album, similar to 1, different vendor, no Intercept
- Intercept Tri Fold, open coin slots: cover folds onto itself.
Intercept Technology protected
- Sample
Z Tri Fold, open coin slots: cover folds onto itself. No Intercept
Technology
The
populated books were placed in the test chamber for a 150ppm hour exposure.
Previous work(7) indicated this exposure is
equivalent to average ambient H2S exposure for 10 years. This relationship of
copper sulfide film growth and sulfur gas exposure has been shown to follow the
formula of RCu,i=lCu,i[í] where RCu,i is the rate of formation
of a sulfur-containing corrosion film on copper by species i, [í] is the
atmospheric sulfurous gas concentration, and lCu,i is the pseudo-first-order
rate constant.
For comparative purposes RCu,i
can be approximated for SO2 and CS2 at a total exposure of 100 ppm-h (approximately the total
sulfurous gas exposure that would occur in 10 year in a typical urban
environment). The derived value of í is 4x10-3 nm
ppb-h-1 for H2S.
A
similar relationship exists for silver and sulfur gases with í being a
lower number in that the reaction efficiency of silver is lower than that of
copper(8).
í
Results
Twelve coins were tested in each album and tri
fold. Typical representations of the exposed coins were selected to evaluate.
One nickel and one penny from each was analyzed.
As observed, the
Intercept album and Intercept tri fold performed without visual degradation.
These had Intercept Technology protection. The Sample X album pennies changed
to an overall darker hue, with the nickels shifting to an even yellow tone. The
Sample Y Album pennies sulfided far worse with additional degradation in the
form of blue/black ringing patterns on the outer edge. The nickel shifted from
yellow to a reddish tone. Although overall corrosion had taken place, corrosion
on the Sample Y album coins were heaviest on the side facing the opening.
The Sample Z tri fold was the worst protector causing the penny to form a
blue/black corrosion film, and the nickel to shift completely yellow/red with
bright but speckled areas of blue. In order to quantify the film growth and
surface chemistry the coins were placed in the scanning electron microscope for
sulfur observation and X-ray analysis for elemental mapping by EDXA.
SEM observations were unremarkable except in areas of surface discontinuities.
For example, where the ear of Lincoln of the Sample Y album penny shows
corrosion occurring at the apex of the raised struck outline of the topography.
This is typical of an altered grain boundary which will exhibit more
susceptibility toward corrosion than the surrounding area as seen in previous
work on the Statue of Liberty restoration(9).
Another significantly altered zone
exists on the outer rim areas of all the coins, shown in figure 3. Sulfide
growth is significantly higher than the surrounding surfaces
(8,696).
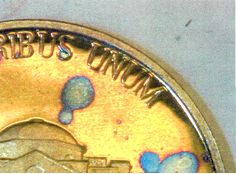
Figure
3 also depicts spot corrosion (blue spots) were created due to localized
increases in time of wetness most likely caused by anhydrous particulates.
These blue areas are indicative of the formation of hydrated sulfate formations
such as posnjakite(10). The remaining
samples did not reveal significant deviations from previous observations.
Further corrosion mapping was deemed unnecessary.
Electron Dispersive
X-Ray Analysis (EDXA) was used to obtain an elemental spectrum of the metal
samples. The evaluation scheme took advantage of the EDXA's ability to
digitally record a background elemental spectrum and subtract that data from
another samples response. The resultant data can then be computed into a ratio
of increase of elements in Thousands of Electron Volts activations (Kev)
to corresponding chemical elements. Since both coins possessed at least 75% Cu
the Cu peak was set as a reference baseline parameter. The La copper
reference peak which is at .93 Kev was used for
reference analysis. The Ka sulfur peak is used as a
corrosivity evaluator. That peak is seen at 0.213 Kev.
The analysis started at zero and stopped counting spectra until 100,000 X-Ray
counts accumulated from the La copper peak. At that time the
Ka sulfur count was recorded.
Data analysis was configured to provide a relative ratio of sulfur
accumulation over the base line as reasonably accurate as possible. The
following analysis remains qualitative in nature. This analysis should not be
considered quantitative.
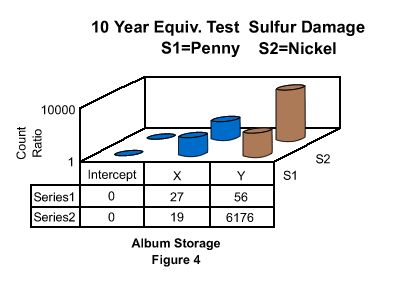
Figure 4 plots the results of the differential
scans of the three types of Album Storage Media coins. The Intercept (Intercept
protected) album sulfur counts were zero and considered baseline. The Sample X
album penny was 27x higher in sulfur and the nickel 19x higher. The Sample Y
album penny was 56x higher in contamination due to sulfur and the nickel 6,176x
higher. In the Tri Fold albums, the penny and nickel samples were at zero for
the Intercept album.
Similar to the previous album the Intercept
protected coin scan was considered at the background level. The Sample Z Tri
Fold is a commodity storage media. Sulfur on the penny stored in the Sample Z
tri fold was measured at a ratio of 6,758 and the nickel at a ratio of
7,257.
Summary
The evaluation of Intercept Technology
encompassed equivalent 10 year sulfurous atmospheric trace gas corrosion
testing followed by optical evaluation, scanning electron microscopy, and x-ray
elemental analysis. This generic testing and evaluation was designed to
demonstrate the protection ability of material packages in reference to
corrosive atmospheric sulfur trace gases and their reactions with copper,
silver, and their alloys. The test results show the tested Intercept Shield
products offer a considerable increase over other non-Intercept protective
products.
References
1) L. Soto, J.P Faney, T.E. Graedel, and, G.W. Kammlott, "On
The Corrosion of Certain Ancient Chinese Bronze Artifacts", Corrosion Science,
Vol. 23, No. 3, pp. 241-250, 1983.
2) P.A. Skiba, Changes in
Reflectivity and Emisivity of Oxide Systems Subjected to the Influence of
Continuous CO2 Laser Radiation, Zhurnal Prikladoni
Spektroskopii, Vol. 37, no. 2, Pages 242-247, Aug., 1982
3) J.H.
Payer, "Corrosion Processes in the Development of Thin Tarnish Films", Dept of
Materials Science and Engineering, Case Western Univ., Cleveland, OH, 1992.
4) T.E. Graedel, J.P. Franey, G.J. Gualtieri, G.W. Kammlott, and D.L.
Malm, "On the Mechanism of Silver and Copper Sulfidation by Atmospheric H2S and OCS", Corrosion Science, Vol 25, No. 12, pg. 1163-1180,
1985.
5) J.P. Franey, T.E. Graedel, and G.W. Kammlott, "The Sulfiding
of Copper by Trace Amounts of Hydrogen Sulfide", The Journal of the
Electrochemical Society, 158th Meeting, Hollywood, FL, 1980.
6) J.P.
Franey, "A Novel System for Atmospheric Corrosion Experiments", Corrosion
Science, Vol. 23, No 1, pg. 1-8, 1983.
7) T.E. Graedel, J.P. Franey,
and G.W. Kammlott, "The Corrosion of Copper by Atmospheric Sulfurous Gases",
Corrosion Science, Vol. 23, No. 11, pg. 1141-1152, 1983.
8) T.E.
Graedel, J.P. Franey, and G.W. Kammlott, "The Corrosion of Silver by Atmosheric
Sulfurous Gases", Corrosion Science, Vol 25, No. 2, pg. 133-143, 1985.
9) J.P. Franey, and M.E. Davis, "Metallographic Studies of the Copper Patina
Formed in the Atmosphere", Corrosion Science, Vol. 27, No. 7 Special Edition,
pg. 659-668, 1987.
10) K. Nassau, P.K. Gallagher, A.E. Miller, and
T.E. Graedel, "The Characterization of Patin Components by X-ray Diffraction
and Evolved Gas Analysis", Corrosion Science, Vol 27, No. 7, Special Edition,
pg. 669-684, 1987.

